San Francisco– The US Food and Drug Administration (FDA) on Monday approved the sleep apnoea detection on the Apple Watch Series 10, Series 9 and Watch Ultra 2.
The FDA’s nod came ahead of the availability of Apple Watch Series 10 from September 20.
The much-anticipated feature was announced at the iPhone 16 launch last week and will arrive as part of the watchOS 11 release.
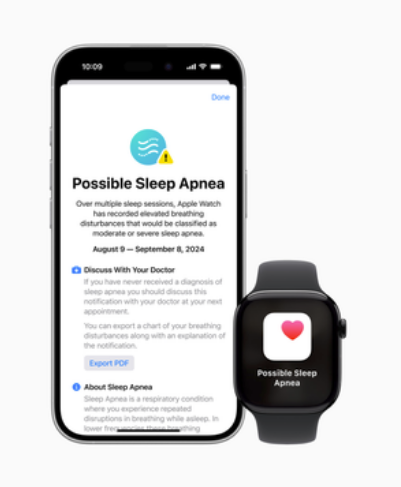
“This device uses software algorithms to analyse input sensor signals and provide a risk assessment for sleep apnoea. It is not intended to provide a standalone diagnosis, replace traditional methods of diagnosis (polysomnography), assist clinicians in diagnosing sleep disorders, or be used as an apnoea monitor,” according to a statement by the US FDA.
The principle of operation is based on analysing physiological signals to assess sleep apnoea.
According to Apple, the feature is not a diagnostic tool but will prompt users to seek out a formal diagnosis.
The sleep apnoea detection feature is a first for the Apple Watch, beginning with the Series 10 model. It will be supported on Apple Watch Series 9, Apple Watch Series 10, and Apple Watch Ultra 2.
According to the tech giant, the sleep notification algorithm was developed using advanced machine learning and an extensive data set of clinical-grade sleep apnoea tests.
The innovative breathing disturbances metric will track users’ sleep, analyse sleep patterns and notify them in the event of an apnoea — a serious sleep disorder where breathing repeatedly stops and starts.
Apple said that the breathing disturbances metric uses the accelerometer to detect small movements at the wrist associated with interruptions to normal respiratory patterns during sleep, and then notify users if it shows consistent signs of moderate to severe sleep apnoea.
The sleep apnoea feature will roll out in 150 countries after its approval from the US FDA. Other standard health features like the Afib alerts, cardio fitness, and the ECG app, from the previous Apple Watch models are also present in the latest model. (IANS)